The first of its kind in Canada
The Biomanufacturing and Industrial Biotechnology graduate program offered at McMaster University is the first academic program of its kind in Canada in an effort to develop a biomanufacturing ecosystem.
The program trains science and technology graduates for high demand leadership roles in this vitally important and rapidly expanding field.
The innovative graduate program combines a foundation in biomanufacturing, bioprocessing, and biopharmaceutical theory with experiential learning in many areas, including cGMP manufacturing, regulatory affairs and biocatalysis.
Curriculum
The interdisciplinary curriculum includes academic courses in science and business, along with professional training through case study projects.
The program also includes professional skills training in effective oral, electronic, and written communications for futures in both technical and business careers. Biomanufacturing and Industrial Biotechnology students will develop and run live upstream and downstream bioprocessing projects to completion, using industry-standard equipment in a simulated cGMP environment.
These projects will provide an insight in advanced cell therapeutics, novel vaccine design and large-scale production, as well as in the standardization of biomanufacturing processes
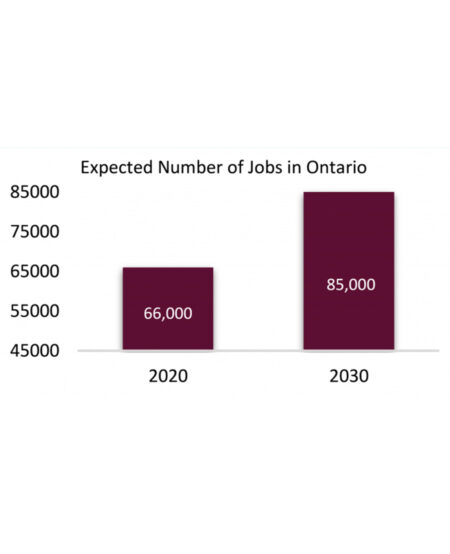
A growing industry
During the start of the COVID-19 pandemic, Ontario was in a perilous position, which forced them to depend on external sources to supply critical goods, mainly the mRNA vaccines produced by American companies Moderna and Pfizer. The lack of innovation in the Ontario health care sector was also seen.
Although Ontario, along with the rest of Canada, was ill-prepared for dealing with the pandemic, the lack of infrastructure and innovation was not always the case. Canada has an impressive history which can be seen from the hundred year-old discovery of insulin to the scientists who were essential in the development of polio and ebola vaccines. In order to have the ability to manufacture our own vaccines and develop innovative solutions, the biomanufacturing sector needs to be built correctly. A biomanufacturing ecosystem in Ontario requires engagement from academic, investor and industry networks to survive and grow